SIDE-EFFECTS OF
HYPERBARIC OXYGEN THERAPY:
EARS
Middle ear barotrauma is the most common side effect of hyperbaric oxygen (HBO2) therapy. An incidence of approximately 2% was found in a retrospective review that included a military population of 1,446 patients who received a total of 31,599 therapies. However, a prospective study using a sensitive method for detection of eustachian tube dysfunction revealed a much higher incidence. After the initial hyperbaric oxygen therapy, 15 of 33 patients (45%) had objective evidence of eustachian tube dysfunction. Not surprisingly, patients who had a history of eustachian tube dysfunction had similar problems during the therapy series. It is likely that the incidence of middle ear barotrauma during hyperbaric oxygen therapy can be highly influenced by factors such as the previous experience of the patient, amount of effort invested in teaching techniques for opening the eustachian tubes, vigilance of the inside medical attendant, and the use of tympanostomy tubes for patients who cannot equalise pressure in their middle ear spaces. Pseudoephedrine has been shown to be effective in preventing barotitis media in a double-blind, randomised, controlled clinical trial in 116 divers. However, a similar controlled trial in 60 patients receiving hyperbaric oxygen therapy failed to demonstrate significant differences in incidence or severity of middle ear barotrauma in two groups of 30 patients who received either topical nasal oxymetazoline hydrochloride or sterile water prior to compression.
SINUSES
Sinus squeeze occurs less frequently than middle ear barotrauma. This second most common therapy-related complication usually occurs in patients with upper respiratory tract infections or allergic rhinitis. Usually a program of decongestant nasal spray, antihistamines, and/or steroid nasal spray just before compression allows therapy to continue.
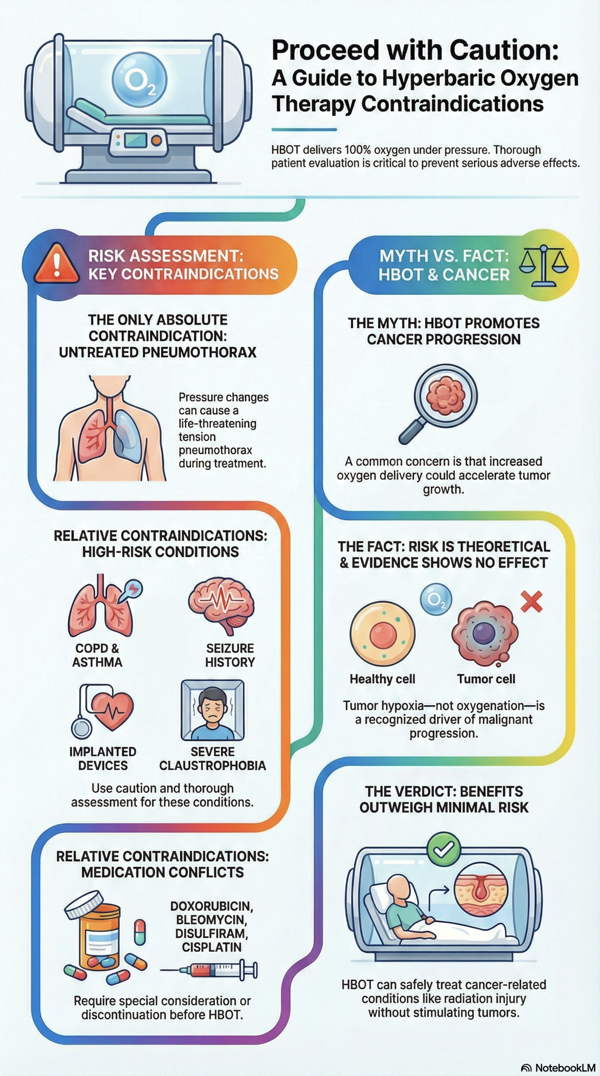
Claustrophobia
Claustrophobia, which appears to be present in about 2% of the general patient population, may cause some degree of confinement anxiety, even in a multi-place chamber. Occasionally, mild sedation is required for such individuals to continue to receive daily HBO2 therapy.
Eyes
Progressive myopia has been observed in some patients undergoing prolonged periods of daily HBO2 therapy. Although the exact mechanism remains obscure, it is apparently lenticular in origin and usually reverses completely within a few days to several weeks after the last therapy. Lyne studied 26 patients undergoing HBO2 therapy for more than a month. Their ages ranged from 36 to 80 yr, and four were diabetics. Treatments were at 2.5 ATM ABS with a 30-min compression while breathing oxygen, 60 min on oxygen at 2.5 ATM ABS, and a 30-min decompression breathing oxygen. Duration of the therapy series ranged from 4 to 52 wk. Pretreatment and monthly follow-up studies included refraction with and without cycloplegia, keratometry, tonometry, fundus examination, and axial length by ultrasonography. Eighteen of the 26 patients, including all 4 diabetics, developed myopia ranging from 0.5 to 5.5 diopters. After the series of HBO2 therapies was concluded, reversal of myopia was usually rapid for the first few weeks and then continued more slowly for periods ranging from several weeks to as long as a year. No other ocular effects were found. Specifically, there were no fundus changes. No patient who started with a clear lens developed any opacities. In those patients with lens opacities present at the beginning of therapy, the opacities did not progress during therapy. No further changes were found during follow-up periods ranging from 6 mo. to 2 yr. Lyne suggested that the change in refraction was due to an increased refractive index of the lens.
Twenty-five patients undergoing extremely prolonged treatment (150-850 daily exposures at 2-2.5 ATM ABS, 7 days a wk) were studied in Sweden. All patients but one showed myopic refractive changes. Of 15 patients with clear lens nuclei before treatment, 7 developed well-defined nuclear cataracts. The earliest that any lenticular change occurred was at 150 treatments over 4 months. Three of the 15 developed nuclear turbidity at 150-200 treatments; 11 developed these changes after 200-850 treatments over 8-19 months; and in one patient no lenticular changes were noted. The nuclear cataracts were not reversible after cessation of these extremely prolonged therapy series.
In what appears to be an exception to the general observation that new cataracts occur only in extremely prolonged series of hyperbaric oxygen therapies, early cataract development in a 49-year-old woman who had only 48 therapies over a period of 11 weeks has been reported. Each therapy consisted of O2 breathing at 2.5 ATM ABS for 90 min with two 5-min air breaks. Bilateral cataract formation was associated with a myopic shift that remained progressive for over 4 months after cessation of the therapy series and then stabilised at 3.25 diopters. The cataracts and associated myopic shift persisted for at least 11 months post-therapy. Although the patient was not diabetic or taking steroids, her unusual susceptibility to cataract formation raises the possibility of an undetected, predisposing condition.
The above published reports, as well as extensive clinical experience in major hyperbaric centres, indicate that, with one possible exception to date, new cataracts do not develop within the series of 20-50 therapies that are commonly used in the United States. Even when progressive myopia does occur, the visual acuity changes almost always reverse completely after cessation of the therapy series. However, extension of a series beyond 100 therapies is associated with an increased risk of irreversible, refractive changes or the development of new cataracts.
Patients should be provided with this information as part of their informed consent for HBO2 therapy. A baseline ophthalmology examination is suggested to detect preexisting lenticular opacities, especially in patients who have conditions associated with an increased risk for cataract development (over 50 years of age, diabetes mellitus, irradiation therapy of the head and neck, and systemic steroid therapy).
Evanger et al, compared refractive changes in 20 patients who received oxygen via a hood system with those in 12 other patients for whom an oro-nasal mask was used. The hood group had a higher incidence of myopia, larger refractive changes, and required a longer period for reversal of the visual changes after cessation of therapy. The investigators concluded that oxygen delivery across the cornea as well as by the arterial circulation caused patients in the hood group to receive a more toxic oxygen dose. This conclusion is supported by the observation in rabbits that PO2 of the aqueous humour was increased significantly by exposing the cornea to oxygen while the animal continued to breathe air. Results of recent studies are also consistent with the possibility that the incidence of myopia may be greater for HBO2 therapy at 2.4 than at 2.0 ATM ABS.
Other reversible effects of hyperoxia on visual function in man include contraction of peripheral vision and reduction in the electrical response of retinal glial cells to a light flash. These effects occur only in oxygen pressure-duration combinations that greatly exceed all but the most aggressive, current applications of HBO2 therapy. Exceptions to this general rule include the development of retrolental fibroplasia (now called retinopathy of prematurity) following exposure of the premature retina to relatively low levels of hyperoxia and a reversible loss of vision during a relatively brief oxygen exposure in an individual who had a previous history of retrobulbar neuritis.
LUNGS
Pulmonary and neurological manifestations of oxygen poisoning are often cited as major concerns. Oxygen tolerance limits that avoid these manifestations are well defined for continuous exposures in normal men. Pulmonary symptoms are not produced by daily exposures to oxygen at 2.0 or 2.4 ATM ABS for 120 or 90 min, respectively. Normal human subjects and patients with significant pulmonary dysfunction (adult respiratory distress syndrome) treated with HBO2 did not demonstrate alterations in pulmonary gas efficiency (pre- and post-HBO2 arterial/alveolar ratio did not change). However, it is possible that cumulative effects of pulmonary oxygen toxicity are produced by repeated daily exposures that individually have no detectable influences on pulmonary function. Statistically significant, but quantitatively small, changes in lung expiratory function were measured in a group of 20 patients after 2-3 weeks of breathing oxygen at 2.4 ATM ABS for 90 min each day. These changes were not associated with pulmonary symptoms or functional limitations. Similar pulmonary function measurements at weekly intervals over a period of 6 weeks did not detect significant changes in a different group of 18 patients who breathed oxygen for 90 min at 2.4 ATM ABS for a total of 30 therapies. The former group of patients had normal lungs and were treated on 21 consecutive days, while the latter group had a preexisting impairment in carbon monoxide diffusing capacity and were treated 5 days each week. Additional measurements are needed to investigate the possible occurrence of cumulative effects during multi-week periods of therapy.
Pulmonary barotrauma with or without associated air embolism may rarely occur during decompression. Patients with airway obstruction are at an increased risk for pulmonary barotrauma during decompression. Significant air trapping and a history of spontaneous pneumothorax are also causes for concern that mandate a careful analysis of potential benefit from hyperbaric oxygen therapy versus the associated risk.
Cardiovascular responses to hyperbaric hyperoxia include a rate-dependent reduction in cardiac output and systemic vasoconstriction with an increase in peripheral vascular resistance. Although these effects are well tolerated by normal individuals, the occurrence of acute pulmonary oedema in three patients during hyperbaric oxygen therapy, with one related fatality, has been reported. All three patients had cardiac disease with reduced left ventricular ejection fractions. The risk of pulmonary oedema may have been increased by the fact that they remained supine in a monoplace chamber during treatment. The present inability to identify patients who are unusually susceptible to this rare complication requires a comprehensive risk-benefit assessment when evaluating patients with compromised cardiac function.
Brain
Early estimates of the seizure rate during therapeutic oxygen exposures at 2.0-3.0 ATM ABS reported a convulsion incidence of about 1 per 10,000 therapies or 0.01%. More recent surveys that include a total of 138,968 patient therapies at three different facilities indicate a combined incidence of about 0.03% for a therapy protocol consisting of three 30-minute periods of oxygen breathing at 2.4-2.5 ATM ABS. Oxygen was delivered by head hood in the recent surveys, while the earlier studies employed a variety of delivery systems that were not always specified. Smerz observed a seizure incidence of 0.6% in a group of 998 patients who received a total of 2166 therapies for decompression sickness at peak pressures of 2.6-2.9 ATM ABS. Apart from the expected influence of a higher treatment pressure, possible reasons for the differences in observed seizure rates include the potential for CO2 accumulation in a hood and unrecognised air leaks allowed by a poorly fitting face mask.
Among 900 patients who received HBO2 therapy for carbon monoxide poisoning, 16 or 1.8% had seizures. The seizure incidence for different O2 pressures ranged from 0.3% at 2.4 ATM ABS (N=300) to 2.5% at 2.8-3.0 ATM ABS (N=600). Even when oxygen convulsions do occur, there are no residual effects if mechanical trauma can be avoided.
The United States Air Force School of Aerospace Medicine did a long-term follow-up study of 563 patients each of whom had over 20 daily HBO2 therapies of 90 min oxygen breathing at 2.4 ATM ABS. The follow-up period was 6 mo. to 8 yr. No chronic or late effects due to HBO2 were seen. Cataracts occurred in only two patients (a poorly controlled diabetic and a 67-yr-old man on high dose steroids).
It has been demonstrated that critically ill paediatric patients can be safely administered HBO2 in a multi-place chamber by experienced personnel who use appropriate precautions. A group of 32 children, ranging in age from 3 days to 11 years, were mechanically ventilated while receiving HBO2 therapy for necrotising infections (N=21), carbon monoxide poisoning (N=9), or iatrogenic arterial air embolism (N=2). Complications included hypotension (63%), bronchospasm (34%), blood behinf eardrum (hemotympanum) (13%), and progressive hypoxaemia (6%). One child was accidentally extubated during transport.

Gregory Weir Vascular Surgery
Vascular & Hyperbaric Unit
Life eugene Marais hospital
The purpose of this web site is to offer Dr Weir’s patients and their families access to information regarding hyperbaric oxygen therapy, wound care and vascular disease in general as well as specific information on certain disease processes. The information on this site does not necessarily apply to all patients with the same diagnosis. If you are not a patient of Dr Weir, please do not regard the information on this website as a substitute for a thorough assessment by a qualified Vascular Surgeon. If in doubt, consult your doctor.
